Leveraging in vivo imaging in zebrafish to understand the genetic basis of adipose morphology
James E. N. Minchin
BHF Centre for Cardiovascular Science, University of Edinburgh, UK
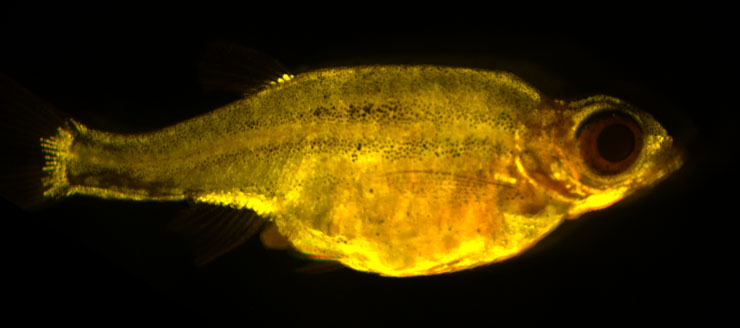
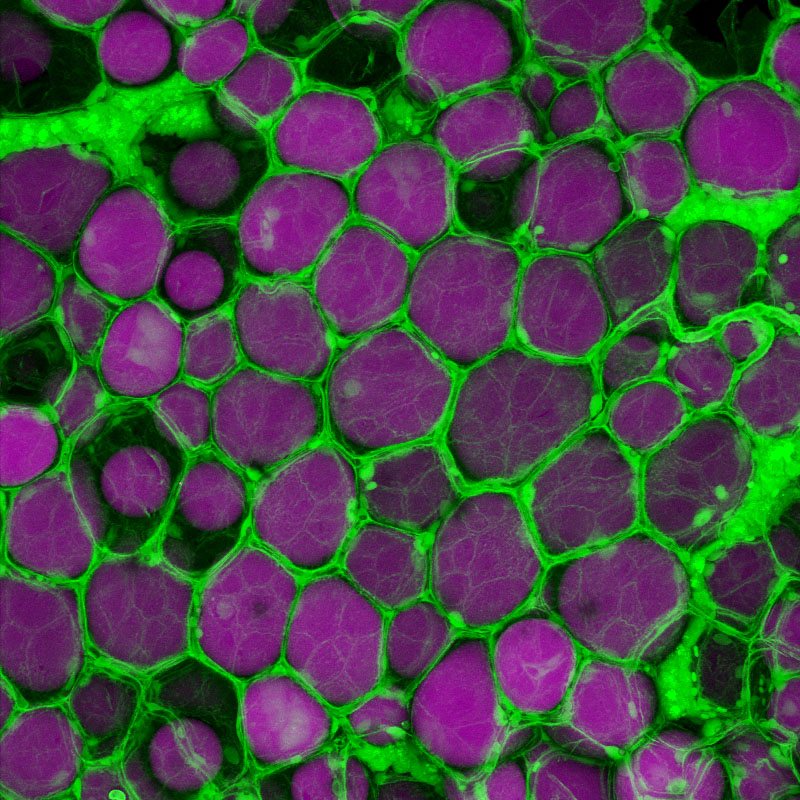
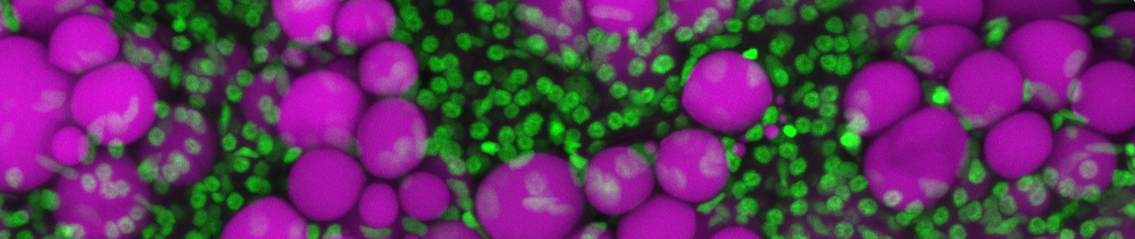
Adipose tissues are highly dynamic lipid-rich structures that possess an extraordinary capacity to expand and contract throughout all life-stages. Adipose tissue expands via two mechanisms; hypertrophy (growth of existing adipocytes) and hyperplasia (addition of new adipocytes). The balance between these distinct mechanisms of growth, and the resulting adipose ‘morphology’, has important health consequences; hypertrophic adipose (containing few, large adipocytes) is associated with increased risk for diabetes and cardiovascular disease. Whereas, hyperplastic adipose (characterized by many, small adipocytes) protects from these diseases. Although adipose morphology is of biomedical importance, the genetic basis that regulates morphology is largely unknown. To study the genetic basis of adipose morphology we have established a zebrafish model that facilitates high-resolution in vivo imaging of adipose tissue growth. Here we describe our data that identifies novel patterns of adipose growth, and new genetic factors that regulate adipose morphology.




You must be logged in to post a comment.